Exploring the Molecules of Life: Water, an Essential Ingredient
What happens to a piece of lettuce that sits out at room temperature for a long period of time? Why?
Raisins come from grapes. How?
What compound is the greatest percentage of essentially all living biological materials?
Water is the major component of essentially all living biological materials. Water typically makes up 50-70% of the mass of the human body. The table below gives the percentage of water in a variety of biological materials.
|
Biological Material |
Percentage of water |
|
Bananas |
76% |
|
Lettuce |
96% |
|
Grapes |
82% |
What is the major use of water in the human body?
Water is the solvent for the biochemistry of life. Materials are transported in it, chemical reactions occur in it, and waste products of cellular metabolism are removed in it. It is pumped as blood through out the body by the heart, filtered by the kidneys, and serves as a reactant and produced as a product in many biochemical processes. This activity will introduce you to the structure and properties of water.
The structure of the water molecule, H2O, is given below. If you place the cursor on the molecule and click you can move it around. Describe the shape of the molecule.
Right click, select Display from the menu, and change it to space fill and van der Waals radius. This will give you a more realistic picture of the water molecule.
The next box shows a cluster of water molecules in the liquid state. View the cluster and which type of nearest neighbors do you find- H to H, H to O or O to O? Why?
Water is a polar molecule, with the oxygen (red) being the negative area and the hydrogen (white) being the more positive area. In ice, solid water, the arrangement is very highly organized (see below).
The molecules of water themselves are held together by covalent bonds between the hydrogen and oxygen. While in ice or even liquid water, the molecules are bound to one another by hydrogen bonding, a strong intermolecular force between hydrogen and highly electronegative elements such as fluorine, oxygen, or nitrogen.
Looking at the ice structure, measure the distance in a O-H covalent bond and in the O.....H of the hydrogen bond (see the Chime Guide for help). The shorter a bond, the stronger the force of attraction. How would you characterize a H-bond compared to a covalent bond?
Hydrogen bonding is very important as an attractive force especially in proteins and nucleotides. The hydrogen bond, a strong intermolecular force, is about 10% the strength of a covalent bond. Water also hydrates ions and other molecules due to its polar nature.
Here is the chloride ion, Cl-, a common ion in blood and other cellular fluids.
How would you describe the orientation of the water molecules surrounding, hydrating, the chloride ion?
Would the arrangement of the water molecules be the same around a potassium ion, K+? Explain why or why not.
The water molecules orient their positive ends, the hydrogens, toward the negative chloride ion, while they would orient their negative ends, the oxygen, toward the positive potassium ion. The ions are hydrated or surrounded by an organized layer of water molecules. The polar nature gives water the amazing power to dissolve most ionic salts, such as sodium chloride or potassium iodide. The polar nature of the water molecule can be seen in the electrostatic potential image below. Red is negative, while blue is positive.
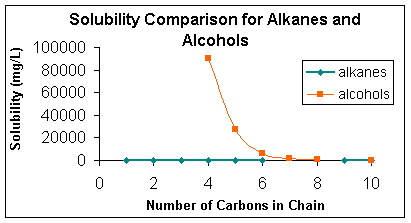
Let's examine the aqueous solubility of some other compounds. The table below gives the solubilities in water at 20oC of two groups of organic compounds as the length of the carbon chain increases. The data are displayed as a graph as well.
|
Number of Carbons in Chain |
Alkane |
Solubility (mg/L) |
Alcohol |
Solubility (mg/L) |
|
1 |
24 |
infinite (can mix in all proportions) |
||
|
2 |
60 |
infinite |
||
|
3 |
62 |
infinite |
||
|
4 |
61 |
90000 |
||
|
5 |
40 |
27000 |
||
|
6 |
10 |
6000 |
||
|
7 |
-- |
1800 |
||
| 8 |
-- |
540 |
||
| 9 |
0.001 |
-- |
||
| 10 |
0.0001 |
0 |
How do the solubilities compare for the alkanes, simple hydrocarbons, to the alcohol of the same carbon chain length?
How would you characterize the solubility of the alcohols compared to the alkanes?
As the carbon chain length increases down the table, how does the solubility of the alkanes behave? How does the solubility of the alcohols behave?

The addition of the -OH onto the alkane to make the similar chain alcohol, changes the non-polar alkane into a polar alcohol. The increase in chain length on the alcohol slowly decreases the polar nature, and hence decreases the solubility. This type of behavior is important in how lipids or fats and oils behave.
Glucose, blood sugar, (on the left) is very water soluble, but cyclohexane (on the right) is not. Explain.
A great site for information on water and its properties is given at Martin Chaplin's - Water: its structure and importance.
Return to Exploring the Molecules of Life homepage.